Osteocera™ Bone Graft Substitute
Osteocera
Osteocera™ Bone Graft Substitute is bioresorbable and can be gradually absorbed and replaced by bone tissue. This product provides a bone void filler that resorbs and is replaced by bone during the healing process.
INTRODUCTION
Osteocera™ Bone Graft Substitute
Osteocera™ Bone Graft Substitute is fabricated from non-living raw materials through a high sintering process, and it is consisted of beta-tricalcium phosphate (β-TCP) with excellent biocompatibility and osteoconductivity.
Osteocera™ is bioresorbable and can be gradually absorbed and replaced by bone tissue. It is a porous structure with a porosity of more than 70%, and it has the highest porosity among synthetic bone graft substitutes. Also, the pores are interconnected with a pore size of 300~600μm; they can conduct bone tissue to grow inside the pores to achieve the purpose of bone repair.
Indication
Osteocera™ Resorbable Bone Substitute is intended for use as a bone void filler for voids or gaps that are not intrinsic to the stability of the bony structure.
Osteocera™ Resorbable Bone Substitute is indicated for use in the treatment of surgically created osseous defects or osseous defects resulting from traumatic injury to the bone.
Osteocera™ Resorbable Bone Substitute is intended to be packed into bony voids or gaps of the skeletal system as a bone void filler (i.e., extremities, spine and pelvis).
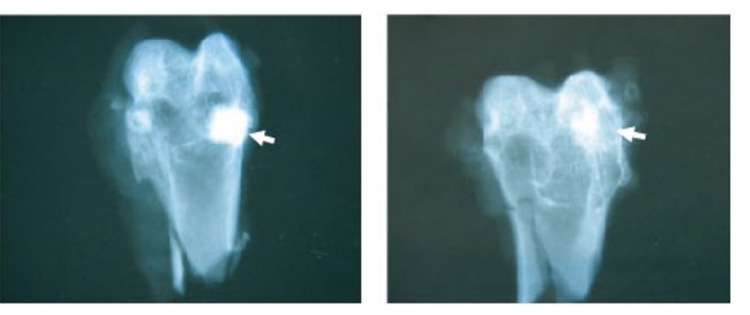
The radiological evaluation shows the remodeling process.
(Left: 1 month; Right: 6 months)
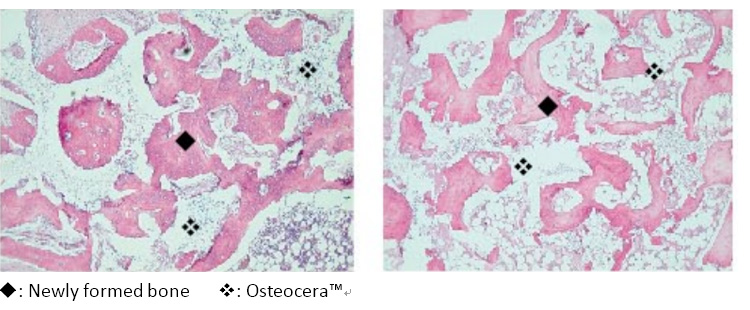
Osteocera™ integrating with the surrounding bone tissue, gradually being replaced by new healthy bone. (Left: 1 month; Right: 6 months)
FEATURES
- Synthetic bone graft with highest porosity
- Non-living raw materials
- Composed of calcium phosphate – excellent reliability and biocompatibility
- Easy for clinical operation
- Biodegradable – can be gradually absorbed and replaced by bone tissue
- Safe and reliable with high quality control
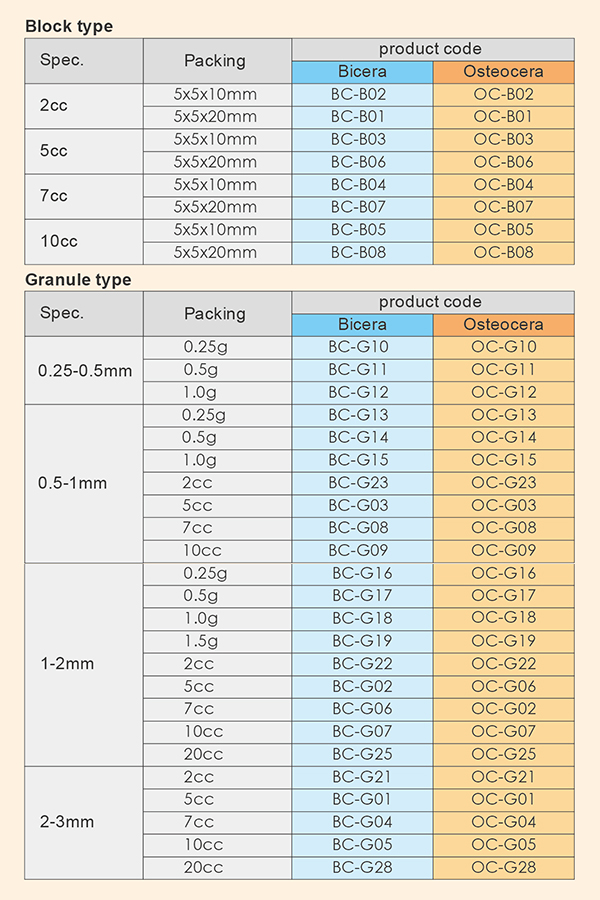
SPECIFICATION
Welcome OEM/ODM, Distribution and Agency Partnerships
For any further question ( Minimum Order Quantity, Prices or Domestic Distributor), please fill out the inquiry form, we will get back to you as soon as possible. Let us work together to make your product come true and hit the market successfully.
- Related Products

BICERA Bone Graft Substitute (HAP TCP)
Bicera
. Composed of HAP (60%) and ß-TCP (40%) bioceramic which is similar to bone mineral . ß-TCP can be gradually absorbed and replaced by bone tissue, and HAP can have a bioactive bonding with new bone to ultimately be part of the bone. . High porosity > 70% porous structure. . Micro pore sizeof 300~600 µm for excellent osteoconductive and bone repair. . Micro pore size of < 10 µm. . High quality with excellent biocompatibility and osseointegration.
- Files Download
 English
English