HI-MUPRO Wound Hydrogel Earns TFDA Class II Medical Device Certification
2025/11/6 Hannox— A Sterile, High-Safety Hydrogel Designed for Chronic and Moderate Exudative Wounds —
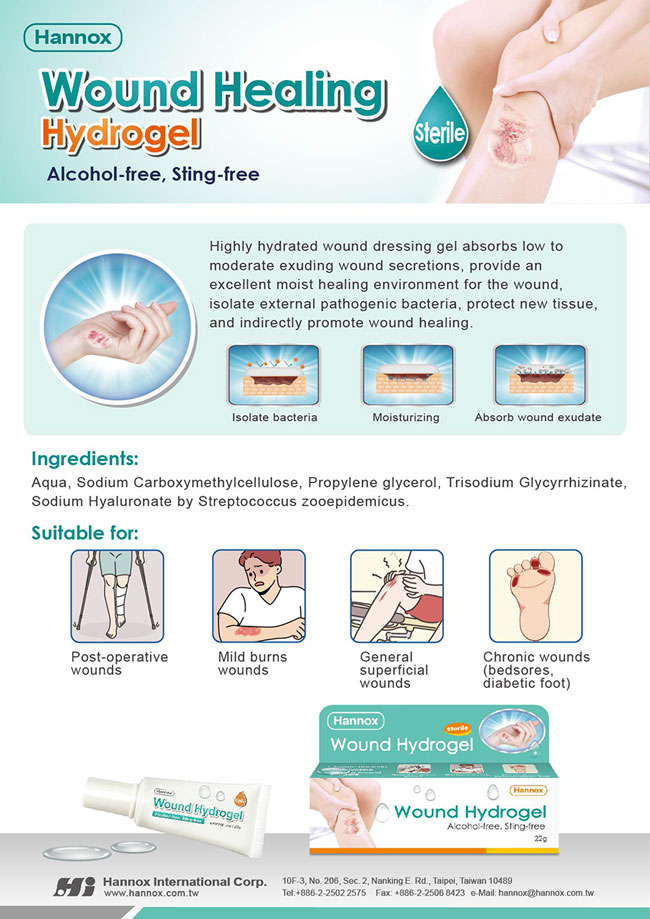
Hannox's new product HI-MUPRO Wound Hydrogel, has officially received TFDA Class II Medical Device Certification. This sterile wound dressing offers enhanced safety and is specially formulated for low to moderate exudative wounds.
HI-MUPRO Wound Hydrogel can be applied to wounds of various shapes and areas of the body. It effectively forms a protective barrier against pathogens, maintains optimal wound moisture, and promotes natural healing. Beyond common abrasions, cuts, and mild burns, it is particularly suitable for chronic wound management, such as pressure ulcers and diabetic foot ulcers.
Key ingredients include Aqua, Sodium Carboxymethylcellulose, Propylene Glycol, Trisodium Glycyrrhizinate, and Sodium Hyaluronate, offering gentle yet long-lasting hydration and protection.
This certification reinforces the company's commitment to scientific innovation and patient safety. The brand will continue expanding its medical wound care portfolio to deliver trusted, effective solutions for both clinical and home use.
- Related Products
Wound Hydrogel
HX-WH22
Hannox Wound Hydrogel is a sterile, amorphous dressing, not only can absorb mild to moderate wound exudate, isolate external bacteria, and provide a moist environment for the wound to protect new tissue and promote wound healing. Hannox Wound Hydrogel is particularly suitable for chronic wounds (bedsores, diabetic foot), Post-operative wounds and mild burns and scalds. Hannox Wound Hydrogel alcohol-free, non-irritating and can provide good wound management.
 English
English